With the advent of continuous and semi-continuous manufacturing in the pharmaceutical industry, companies are looking for solutions to their reporting, analytics, and materials traceability needs. Many processes that have been manufactured using traditional batch techniques are now evolving towards continuous manufacturing in order to improve manufacturing efficiency and product uniformity.
InfoBatch for Continuous Manufacturing
For example, there is intense research and development associated with applying continuous process technology to drug manufacturing that was previously batch-oriented. The regulatory and traceability considerations are particularly critical in this area.

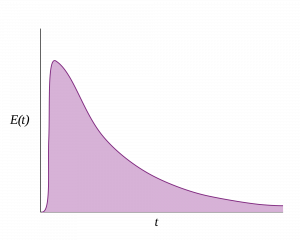
Figure: Residence Time Distribution for an ideal continuously-stirred tank reactor.
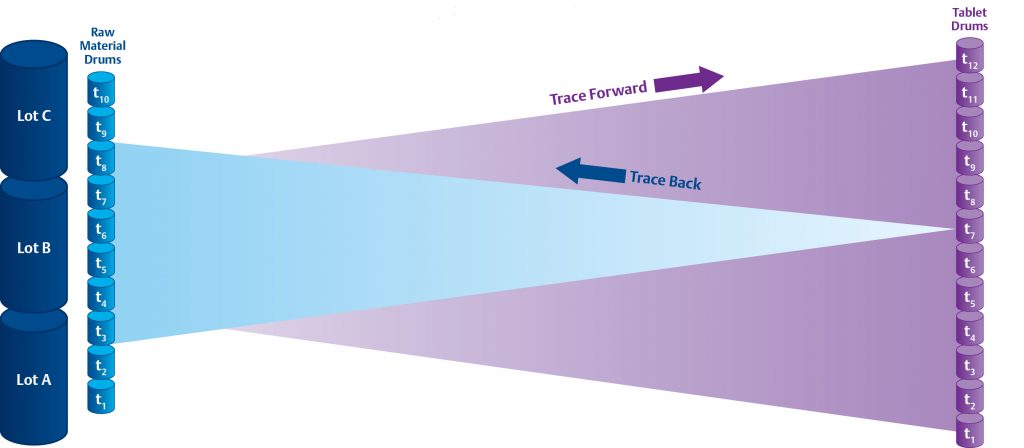
InfoBatch® aggregates and contextualizes manufacturing data to facilitate reporting and analysis. In a batch process, InfoBatch utilizes existing recipe time stamps to trace material and contextualize process events and conditions.
In a continuous process however, process steps have no definitive time stamps, and so InfoBatch takes a model-driven approach. Each process step, or Unit Operation, has a Residence Time Distribution (RTD) which describes the range of times and concentrations that it takes batch material to pass through the Unit Operation in a steady-state.
Using the RTD’s of successive Unit Operations, InfoBatch can trace material through your process in either direction.