Browse our collection of whitepapers, case studies, videos, and more, written by industry experts.
All Resources
The Problem: When Isolated Data Needs to be Brought into Compliance Manufacturing equipment produces huge amounts of data Read more
Members of the manufacturing and quality departments know that properly managing all of the documents associated with the Read more
Those who have been tasked with documentation lifecycle management for highly regulated industries know what a daunting task Read more
Curious about our new InfoBatch® InfoETL solution but unsure what "ETL" actually means? We've written a brief guide Read more
Both American and European regulatory bodies publish guidelines for Good Manufacturing Practices that detail proper protocol for pharmaceutical Read more
Logbooks are utilized extensively throughout manufacturing operations to capture records of operator activity and critical process data. These records Read more
About this video: Follow along as we use RQMS™ to interactively slit a master roll. Video Transcript: This Read more
Event Monitor (EM) triggers InfoLog® events from external manufacturing systems. EM provides a service to monitor events from Read more
About this video: Watch this video to learn how how InfoLog®, Informetric's electronic logbook application, can be used Read more
Blog
The Problem: When Isolated Data Needs to be Brought into Compliance Manufacturing equipment produces huge amounts of data Read more
Members of the manufacturing and quality departments know that properly managing all of the documents associated with the Read more
Those who have been tasked with documentation lifecycle management for highly regulated industries know what a daunting task Read more
See All
Whitepapers
Tired of incomplete, hard-to-read paper log books? Automate your event logs and integrate them with your control system. Read more
Introduction Manufacturers subject to Good Manufacturing Practices (GMP) are required to document system modifications as well as operational Read more
Traditional Task Management and Logbook Difficulties Every plant has common tasks that ensure day to day operation is Read more
Case Studies
In 2022, a pharmaceutical manufacturer needed a strategy to seamlessly report on active and archived DeltaV™ data. The Read more
A herbicide manufacturer in Louisiana experienced a safety near miss due to miscommunication between shifts. The manufacturer needed Read more
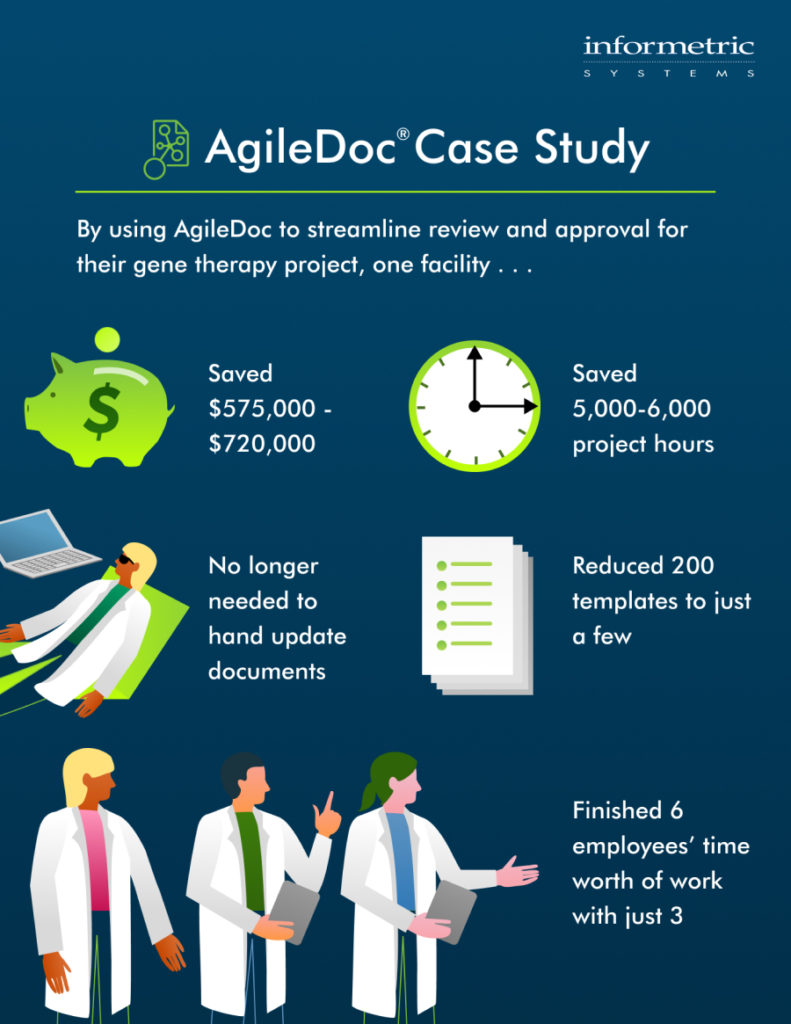
By using AgileDoc to streamline review and approval for their gene therapy project, one facility: If you are Read more
See All
Application Notes
Audit trails provide critical information on configuration changes that potentially affect manufacturing systems and batch release. Audit trails Read more
Need more columns in your report than you can fit on a single, letter-sized page? Want to organize Read more
Event Monitor (EM) triggers InfoLog® events from external manufacturing systems. EM provides a service to monitor events from Read more
See All
Videos
About this video: Follow along as we use RQMS™ to interactively slit a master roll. Video Transcript: This Read more
About this video: Watch this video to learn how how InfoLog®, Informetric's electronic logbook application, can be used Read more
About this video: Follow along as we use InfoBatch® to generate a report and perform a simple configuration Read more
See All