In 2021, a multinational life sciences company with biologics manufacturing facilities in the Bay Area sought software that would match their unique reporting requirements. Their operators needed the ability to...
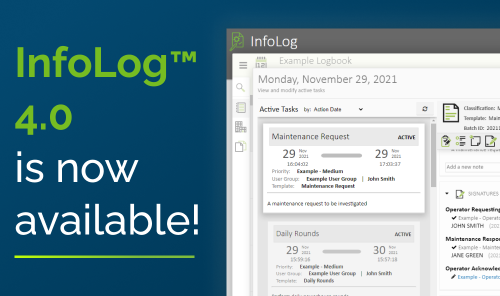
Read MoreInformetric Systems and Emerson Automation Solutions have expanded their partnership with the transfer of Emerson’s DeltaV™ Logbooks and Syncade™ Logbooks to Informetric. InfoLog® 4.0 is the result of that partnership....
Read MoreWe are excited to announce the release of the AgileDoc® Comparison Tool. AgileDoc is a Software Lifecycle (SLC) documentation suite. AgileDoc automatically generates documents from system source code, thus improving speed...
Read MoreIn 2020, a global manufacturer of advanced film products engaged Informetric Systems to deploy RQMS, or Roll Quality Management System. ...
Read MoreLife science manufacturers are mobilizing with unprecedented speed to release effective COVID-19 vaccines. The science behind the vaccines is impressive itself, but the speed and scale with which manufacturers are...
Read MoreInformetric Systems and Emerson are expanding their partnership with the transfer of Emerson’s DeltaV Logbooks and Syncade Logbooks to Informetric. Emerson and Informetric entered into an agreement in which Informetric...
Read MoreInformetric to host interactive AgileDoc Training remotely on October 6 - 8, 2020. Click here to learn more and register. ...
Read MoreAt Informetric Systems Inc. we are determined to support our customers during this tremendously difficult time and are taking generous measures to make sure our customers are fully satisfied while...
Read MoreStreamline compliance with electronic review and approval! AgileDoc® Workflow creates an expansive GMP context-based document review system to safely manage electronic records, attach associated documents and review process history. It provides an...
Read MoreInformetric will be exhibiting at the RE Mason User Exchange on Tuesday, May 12th in Midlothian, VA and Thursday, May 14th in Charlotte, NC. Come learn what's new...
Read More